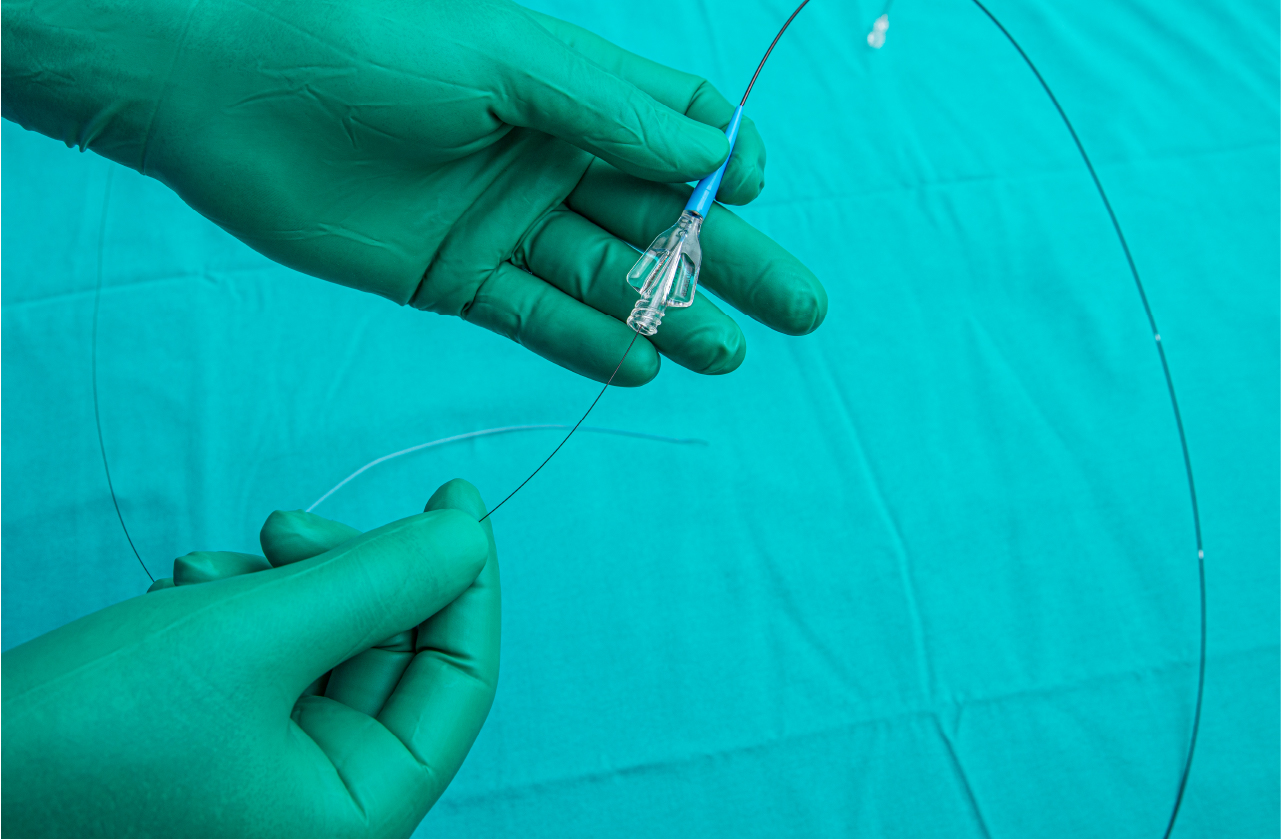
For years, medical professionals have relied on traditional procedures when conducting cardiovascular interventions. Akin to navigating a snake through a clogged drainpipe, use of guidewires and catheters directed by 2D imaging have long since been the norm for cardiovascular clinicians. While such practices are effective, rapid advances in technological capabilities have opened the door to innovations that have the potential to drastically reduce procedure times as well as overall failure rates.
In Canada’s rapidly growing image-guided therapy (IGT) field, Magellan Biomedical is one organization leading the development of technology focused on enhancing current cardiovascular diagnostic and treatment practices. Magellan Biomedical’s technology not only allows clinicians to achieve localized position control of any off-the-shelf guidewire, but also enhances the navigation of such devices by leveraging the power of 3D visualization. While this technology has the potential to be used in interventions across a plethora of diseases, the immediate need addressed by Magellan Biomedical is in therapy of peripheral arterial disease. With roughly 50-80% of typical procedure time dedicated to manipulating the guidewire, such technology has the potential to revolutionize the future of cardiovascular interventions. Adding to the power of this technology, is its seamless fit into current practices. Magellan Biomedical’s technology does not alter conventional procedures but enhances existing practices. While compatible with any off-the-shelf guidewire or catheter, this technology is not only easy to integrate, but also cost-effective.
In an interview with Ali Tavallaei, President and Director at Magellan Biomedical, Tavallaei spoke to altruistic intentions when posed the question of how their career in biomedical technologies began. While as a child, Tavallaei revered the work done by surgeons and clinicians – noting their hand in the immediate relief of patient pain – Tavallaei’s interests aligned closer to engineering. In an attempt to bridge these two passions, Tavallaei pursued a PhD in Biomedical Engineering Science from Western University. While completing a PhD, Tavallaei co-founded Vital Biomedical Technologies which received numerous accolades and grants for its work with MRI compatible actuators. In 2016, Tavallaei joined the Sunnybrook Health Sciences Centre as a Postdoctoral Fellow, continuing work in the advancement of medical technology. With further opportunity on the horizon, Tavallaei accepted a faculty position at Ryerson University in 2019 and there continues teaching and advancing the work of Magellan Biomedical.
When asked about key insights gained from experience in the field, Tavallaei spoke to the importance of building a strong and diverse team, with a complimentary skillset. The Magellan Biomedical team is a mission-aligned group of individuals consisting of engineers, inventors, clinicians, and scientists. With the right team, a strong product, and a growing industry, Magellan Biomedical is uniquely positioned in the marketplace but faces one primary challenge. Given the relatively low profile of Canada’s image-guided therapy industry, Tavallaei noted the difficulty associated with identifying lead investors that have previous experience investing in the space. Thus, the importance of strengthening Canada’s national presence in the field is a critical next step to attracting new investors and seeking continued funding.
While the image-guided therapy space is rich with innovative start-ups, like Magellan Biomedical, it has yet to see the formation of an integrated community that allows for cross-sector collaboration, seamless knowledge transfer, and the amplification of the field’s national and international profile. This presents tremendous opportunity in the space given Canada’s strong foundation in image-guided therapy technology. Tavallaei spoke of Canada as home to some of the “best centers or institutes globally that are active in the medical imaging space”. With pioneering technologies developed here at home, the key opportunity that has yet to be leveraged is how to properly amplify and commercialize these innovations. With such untapped potential, the partnership between Magellan Biomedical and the INOVAIT network is one step forward in bridging the gap between existing innovators in the field and attracting national and international attention from potential investors.