Non-Alcoholic Fatty Liver Disease across North America
Non-alcoholic fatty liver disease is a silent epidemic spreading across North America. It’s estimated that 1 in 5 people in Canada have fatty liver disease. Over the last few decades, the prevalence of this disease has ballooned globally due to dominating sedentary lifestyles and high-fat foods consumed each day, along with other comorbidities that contribute to its progression, including diabetes and obesity. Like with many other life-threatening diseases, early detection and monitoring is crucial to ensuring better treatment outcomes and prognosis.
Introduction to Sonic Incytes
Sonic Incytes, a Canadian artificial intelligence (AI)-assisted medical imaging company based out of Vancouver, British Columbia, is looking to change the way liver disease is screened and monitored globally. Their flagship, FDA cleared device, Velacur™, is revolutionizing the diagnosis and management of chronic liver disease. Their proprietary technology that grew into Velacur was originally developed by researchers at the University of British Columbia (UBC).
Current Fatty Liver Disease Screening Options are Leaving Millions Behind
Fatty liver disease largely goes undiagnosed due to inaccessible screening options. Today, the only ways you can be screened for fatty liver disease are through invasive biopsies, expensive MRI scans, or limited transient elastography. Liver biopsies are invasive and involve the collection of a small tissue sample which only reveals a fraction of the overall liver health. MRIs are used to measure the fat and elasticity in the liver but are quite costly both in time and money and, in Canada, often have long wait times. The primary screening method for fatty liver disease is transient elastography, but similar to a biopsy, this point-and-shoot system only offers a small viewing window into the overall health of the liver. Not to mention, it has a limited measurement depth and volume sample collected. Since the vast majority of high-risk individuals are overweight or obese, transient elastography is often an ineffective screening method. Lastly, all of these screening methods require immense technical training and expertise. To tackle this growing epidemic, we need a new system to help meet the enormous unmet screening and disease monitoring needs. That’s where Velacur comes into play.
An Innovative Solution: Velacur
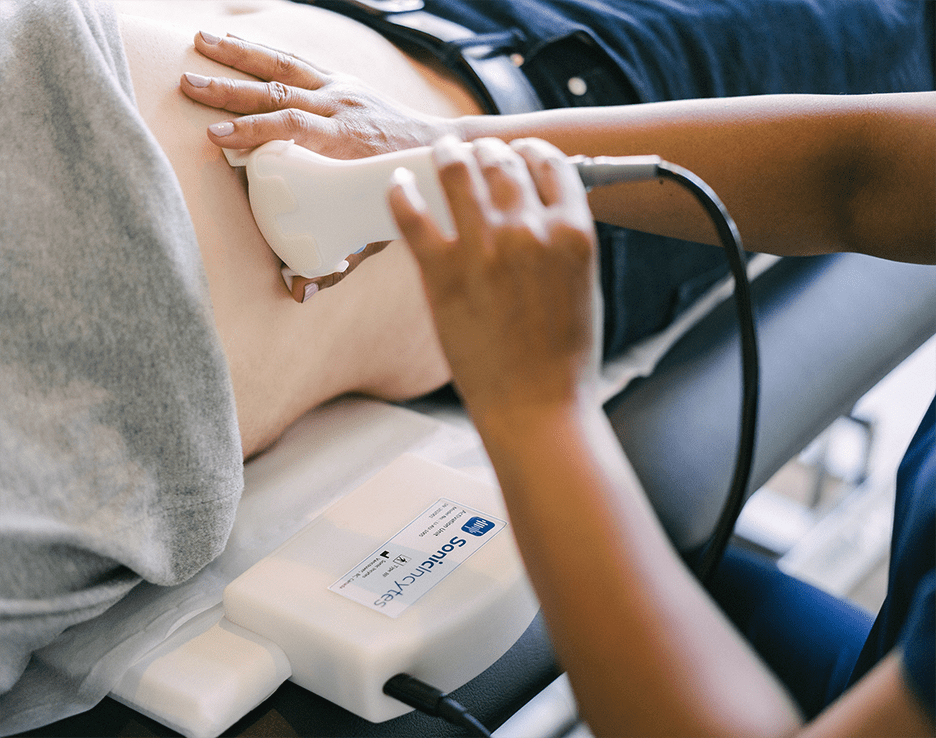
Velacur is Sonic Incytes’ innovative, AI-assisted ultrasound that uses S-WAVE technology to accurately and rapidly measure liver stiffness (fibrosis) and attenuation (fat content), key indicators of chronic liver disease. Unlike other non-invasive technologies, Velacur offers superior point-of-care diagnosis, with an easy-to-use interface enabling accurate scans in just 5 minutes, with no sonography experience needed. Additionally, Velacur’s 3D scanning measures 30x more tissue volume with double the depth, ideal for patients with high body fat percentages.

The team at Sonic Incytes has dedicated themselves to ensuring that Velacur is accessible and affordable, to enhance patient care. “We are passionate about increasing the accessibility of liver screening,” said Barry Allen, CEO at Sonic Incytes. “Through INOVAIT funding, we have enhanced Velacur to assist users in obtaining high-quality liver scans, regardless of patient body type or clinical expertise.”
In 2022, Sonic Incytes was a recipient of the INOVAIT Pilot Fund. Shortly thereafter, their progress on Velacur’s enhancements proved incredibly promising, and they were awarded the coveted INOVAIT Focus Fund to further advance Velacur. Since launching their project, Velacur has vastly improved the user experience for healthcare professionals through the release of new FDA cleared AI features, the Organ Guide and Wave Quality Detector. The Organ Guide integrates an in-house developed AI tool that identifies not only the liver but also other organs, tissues, and artifacts in real time. Additionally, it includes a Wave Quality measurement feature that assesses the quantity and quality of shear waves generated and measured by the device, providing superior measurements for people with higher body fat percentages. These crucial enhancements improve the performance of the device to enable less experienced medical personnel to conduct accurate liver scans, with no sonography experience required. The continuous improvement of an innovative medical imaging device requires the united vision of an extremely skilled interdisciplinary team. With INOVAIT’s funding, Sonic Incytes has created and maintained numerous roles within the company to ensure the streamlined growth of Velacur. The skillful team is hard at work further advancing Velacur to improve patient outcomes and help tackle the fatty liver disease epidemic.
“Today, reading an ultrasound image requires a lot of skill and training,” said Allen. “By assisting the user with our advanced features, we can help less experienced users capture fantastic images, ultimately leading to higher quality data. With machine learning assistance, Velacur will lead to increased accuracy of disease progression.”
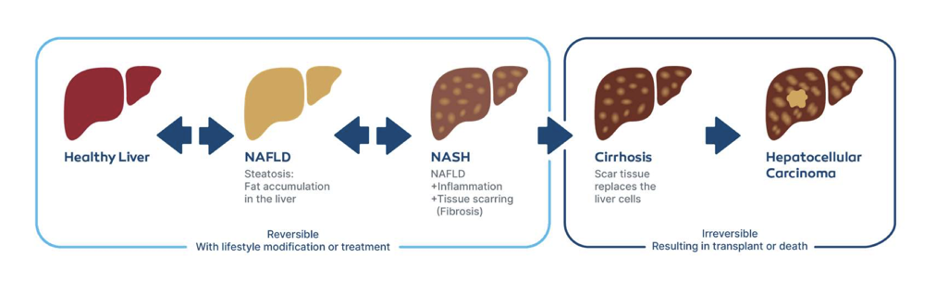
Figure 1: Fatty liver disease progression is considered to have five phases. The first three phases of liver disease are reversible. Early diagnosis and monitoring are key to reversing liver disease progression.
The progression of fatty liver disease is often described in five phases (see Figure 1). The first three phases of the disease are reversible with the right lifestyle changes and treatment. As more pharmaceutical treatments become available for those suffering from fatty liver disease, it will become even more critical to have a reliable system to evaluate the disease progression of the liver on a more frequent basis. Velacur is poised to fill that gap, providing an easy-to-use, portable, point-of-care device that can assist millions of patients.
In summary, fatty liver disease is a growing health concern that affects millions of people in Canada and around the world. The current screening options are either invasive, inaccessible, or lack accuracy, making it difficult to diagnose and regularly monitor the condition. Sonic Incytes, with its Velacur device, has engineered a non-invasive, accurate and accessible liver screening and monitoring device that can help support millions of patients manage their liver disease. With the addition of AI-powered features, this technology that was developed out of a UBC research lab is destined to revolutionize the way liver disease is screened and monitored globally.