PulseMedica aims to transform vitreoretinal disease outcomes in the blink of an eye.
Inside a nondescript office building in downtown Edmonton is an image-guided therapy company hard at work building revolutionary technology that has the potential to significantly impact eye imaging and treatment. PulseMedica, founded in 2020 by Dr. Abdul Elezzabi, Dr. Nir Katchinskiy, and Eric Martin, is poised to revolutionize how many common vitreoretinal eye diseases, like diabetic retinopathy and eye floaters, are identified, monitored, and treated.
From the instant you walk into PulseMedica’s office, you’re transported to a vibrant and buzzing open concept office-lab, designed to support their multi-disciplinary engineers to collaborate and build synchronously in real-time. Its office is no different from a beehive, with each person intensely focused on their work to build this groundbreaking medical innovation. From the whirring of machines and brainstorming chatter, you’re instantly pulled into the excitement of an eager team. With its growing team, PulseMedica is rapidly preparing to launch its image-guided therapy prototype device into the clinic in the coming months to complete their clinical trials.
The PulseMedica platform is a 3D image-guided therapy device that integrates powerful laser therapy, advanced medical imaging, and limitless machine learning (ML) for precision imaging and treatment of vitreoretinal disease. Vitreoretinal diseases exist within the clear gel (vitreous fluid) that fills the eye and retina at the back of the eye. PulseMedica’s device strives to automate the imaging and treatment process, enabling quick and precise disease targeting, thereby reducing the treatment time and reducing the risk of side effects and total time per patient, allowing for more patients to be treated each day.
An electrical engineer by training, Katchinskiy’s and Elezzabi’s vision for their device began with the Femtosecond laser research and development at the University of Alberta. Their research unknowingly led to a groundbreaking discovery in ophthalmic care having discovered a new method for treating vitreoretinal eye conditions with this laser technology. Over the years, ophthalmologists and retinal surgeons have expressed a keen interest in the potential application of femtosecond laser technology beyond its use in LASIK and cataract surgeries, such as for diabetic retinopathy and eye floaters. This enthusiasm stems from the remarkable abilities and precision of the technology.
Diabetic retinopathy and eye floaters
Diabetic retinopathy is the leading cause of vision loss globally. An estimated 95 million people live with some form of diabetic retinopathy. In Canada, most Canadians living with diabetes are 65 years and older. With Canada’s senior population expected to skyrocket 68% over the next 20 years, Canada and the rest of the world, for that matter, are in need of additional imaging and treatment options that can combat diabetic retinopathy.
In addition to diabetic retinopathy, eye floaters are one of the most common eye diseases among older adults. Eye floaters can block or skew a person’s vision leading to issues with quality of life. Up to 70% of people worldwide will experience eye floaters at some point in their life.
The degeneration of vision can lead to a poorer quality of life. Individuals with vision impairment are more likely to experience restrictions in their independence and mobility, as well as an increased risk of falls, fractures, injuries, poor mental health, cognitive deficits and more. The consequences of not treating these eye conditions are extensive. In their current state, global healthcare systems are ill-prepared to handle the volume of needs that grows each day.
Fortunately, PulseMedica is laser-focused on improving the quality of life for millions of people living with vitreoretinal diseases with its real-time 3D imaging and laser targeting system. These technologies have the potential to provide more accurate disease screening and diagnosis while introducing a safe, effective, and non-invasive image-guided treatment procedure.

Femtosecond lasers
The critical quality that has challenged researchers in advancing eye floater treatment is that eye floaters are in perpetual motion. Attempting to treat an eye floater is essentially like trying to shoot a moving target with an arrow.
The advantage of using femtosecond lasers is that they are incredibly fast thereby reducing any surrounding tissue damage- far less than traditional optical lasers. The collateral damage associated with conventional lasers, such as vision loss, is drastically reduced. In the blink of an eye, the PulseMedica image-guided treatment device aims to eliminate eye floaters. With the advent of artificial intelligence consuming the medical community in the last five years, Katchinskiy and his team will be collecting, reviewing, and analyzing tens of thousands of 3D eye images to effectively train their device’s machine learning algorithm to predict the location and movement of floaters accurately. The combination of its device’s medical imaging, machine learning, and the laser will enable increased precision to diagnose, track, prescribe, and execute the appropriate treatment for the patient and deliver it seemingly instantaneously.
INOVAIT Pilot and Focus Fund support
The PulseMedica device represents the trifecta of medical device excellence in 2024; marrying exceptional multi-disciplinary engineering with the limitless capabilities of machine intelligence in hopes to deliver unmatched medical performance.
In 2020, INOVAIT, through Canada’s Strategic Innovation Fund, witnessed potential in PulseMedica’s technology and selected it as one of the recipients of the INOVAIT Pilot Fund to further develop their device’s hardware and software. Beginning with the INOVAIT Pilot Fund project, PulseMedica enhanced its existing systems’ software by integrating ML into its diagnostic tool. Through the intuitive user interface, ophthalmologists, nurses and technologists can interact with patient images and ML-generated detection markers to identify diseases and chart a treatment plan (see image below).
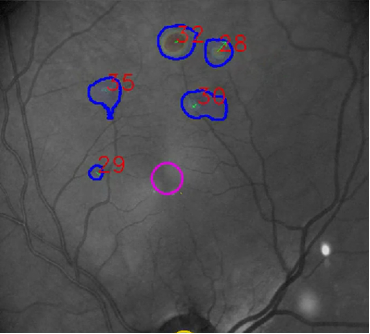
PulseMedica will continually collect user feedback from practicing ophthalmologists to ensure the user interface and workflow reflect the real needs of the clinicians enabling enhanced patient care and clinical outcomes.
Having received Investigational Testing Authorization from Health Canada for its first-in-human clinical trial, PulseMedica is now validating its device’s capabilities to obtain the necessary documentation and approval from Health Canada and, eventually, the FDA to bring the device to market.
Global vitreoretinal disease diagnosis and treatment markets
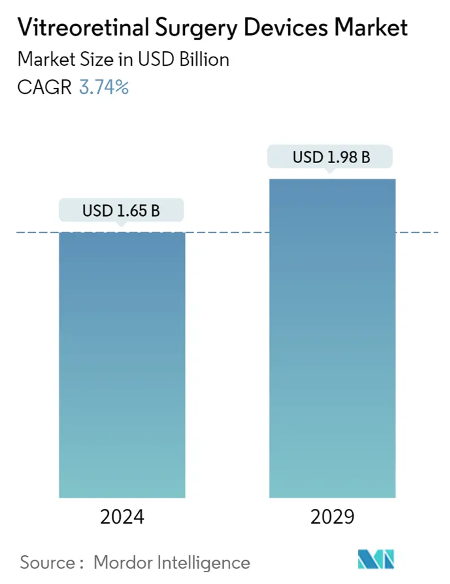
The Vitreoretinal Surgery Devices Market is projected to grow from USD 1.59 billion in 2023 to USD 1.91 billion by 2028. Since the PulseMedica device serves both diagnostic and treatment purposes, it is poised to tap into an even larger market segment than just the surgical devices market. Furthermore, as the global population ages, the market size for such innovative medical devices is expected to increase significantly.
PulseMedica’s journey, fueled by the dedication and expertise of its team, mentors and investors, mirrors the dynamic and collaborative spirit of its office-lab environment. It’s a testament to the power of human ingenuity and technological advancement working in tandem, like a well-oiled machine. The potential impact of its work extends far beyond the walls of their Edmonton office; it reaches into the lives of millions worldwide, offering a new lease on sight and quality of life.
As we look towards the future, PulseMedica’s device is not just an invention; it’s a beacon of hope for improved patient care and a catalyst for change in the medical technology landscape. In the future PulseMedica is not just launching a product; it is charting a new course in the fight against eye diseases. Its commitment to innovation, precision, and patient care heralds a new era in ophthalmology, one where vision loss is no longer an inevitable fate, but a challenge met with the best of what technology and humanity have to offer.
If you want to learn more about PulseMedica and their device, we invite you to visit their website: www.pulsemedica.com